MAGNESIUM
Magnesium is the least active of the alkali metals. It is ductile, malleable and, although white, is easily coated with a layer of white oxide (MgO) which gives the gray metal that we normally see. It is widely used by lightweight alloys for aircraft. Burns easily react with oxygen and nitrogen from the air, why is widely used in fireworks because it creates in the combustion a very intense white light of short wavelength.
Mg + O 2 -> MgO
3 Mg + N 2 -> Mg 3 N 2
Magnesium is the least active of the alkali metals. It is ductile, malleable and, although white, is easily coated with a layer of white oxide (MgO) which gives the gray metal that we normally see. It is widely used by lightweight alloys for aircraft. Burns easily react with oxygen and nitrogen from the air, why is widely used in fireworks because it creates in the combustion a very intense white light of short wavelength.
Mg + O 2 -> MgO
3 Mg + N 2 -> Mg 3 N 2
We will conduct two practices relating to magnesium
Combustion of magnesium

spatula, crucible tongs.
Products:
magnesium. PROCEDURE
Cut 2 cm of magnesium ribbon.
Please scratch this off with a spatula and examines the color and brightness that appears.
Turn it on with the help of crucible tongs.
reaction of magnesium with hydrochloric acid-2 MATERIAL
Crystallizer, gas tube, pipette bulb safety, watch glass.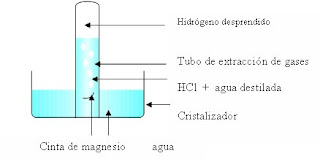
Products:
Magnesium, concentrated hydrochloric acid.
PROCEDURE Put water
halfway into a crystallizer. Full
exhaust tube with water almost completely, and add 5 ml of concentrated hydrochloric acid (pour making sliding down the wall of the tube gases, fume cupboard and under the supervision of your teacher).
Finish filling the tube with water so that the meniscus beyond the edge of the tube, but not dripping.
cover the tube with the bottle, turn it over and insert it in the crystallizer so that it is as follows:
weighs 2 cm of magnesium ribbon and insert it quickly into the gas tube.
expected to complete the reaction and record the volume of gas evolved. Back
cap the gas pipe and remove it from the water. With the tube still blocked on a call and he removes the tube.
See what happens.
Products:
magnesium. PROCEDURE
Cut 2 cm of magnesium ribbon.
Please scratch this off with a spatula and examines the color and brightness that appears.
Turn it on with the help of crucible tongs.
reaction of magnesium with hydrochloric acid-2 MATERIAL
Crystallizer, gas tube, pipette bulb safety, watch glass.

Products:
Magnesium, concentrated hydrochloric acid.
PROCEDURE Put water
halfway into a crystallizer. Full
exhaust tube with water almost completely, and add 5 ml of concentrated hydrochloric acid (pour making sliding down the wall of the tube gases, fume cupboard and under the supervision of your teacher).
Finish filling the tube with water so that the meniscus beyond the edge of the tube, but not dripping.
cover the tube with the bottle, turn it over and insert it in the crystallizer so that it is as follows:
weighs 2 cm of magnesium ribbon and insert it quickly into the gas tube.
expected to complete the reaction and record the volume of gas evolved. Back
cap the gas pipe and remove it from the water. With the tube still blocked on a call and he removes the tube.
See what happens.

0 comments:
Post a Comment